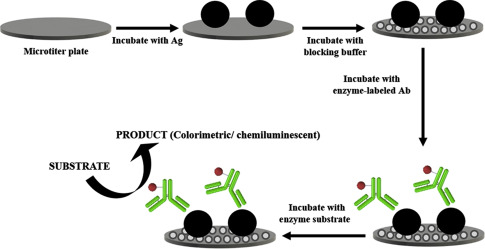
Enzyme-linked immunosorbent assays (ELISA) remain a cornerstone of biomolecular detection, with applications spanning immunology, oncology, neurodegenerative disease research, and infectious disease diagnostics. While conventional ELISA platforms achieve picogram-level sensitivity, emerging methodologies now push detection limits into the femtogram and even attogram range. This article explores the latest advancements in high-sensitivity ELISA, including signal amplification strategies, enzyme engineering, nanoparticle-enhanced detection, and next-generation substrates that collectively redefine the lower limits of biomarker quantification.
Challenges in High-Sensitivity Detection
The primary limitations in conventional ELISA arise from:
- Signal-to-noise ratio (SNR): Background noise from non-specific binding and substrate auto-fluorescence can obscure low-abundance targets.
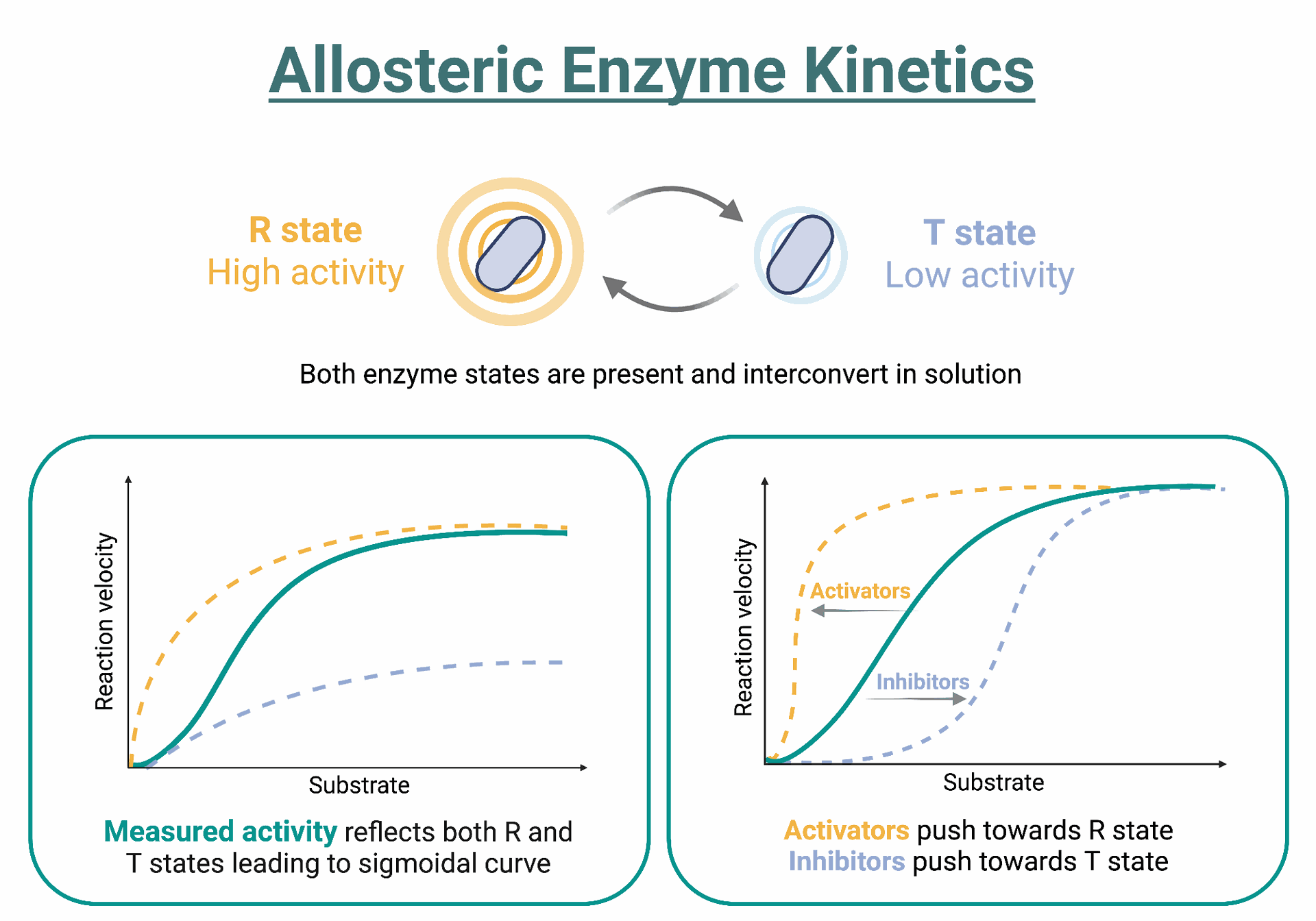
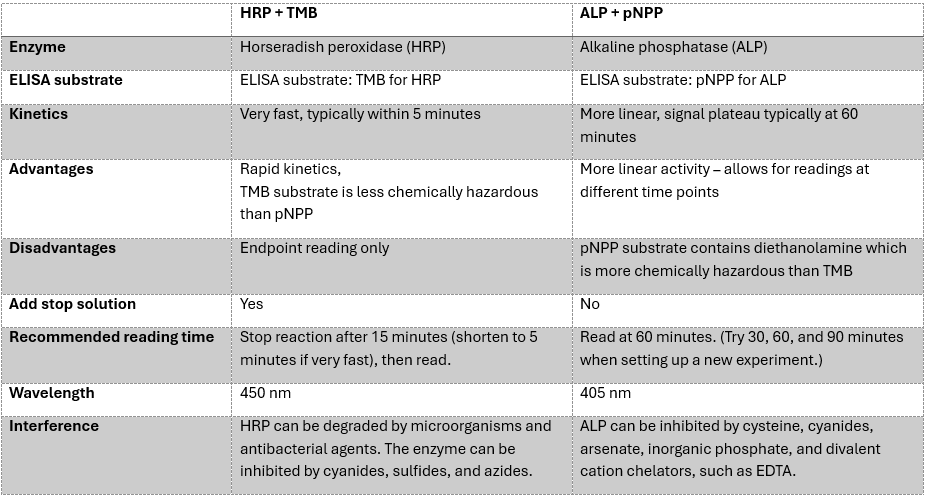
- Enzyme kinetics: Conventional horseradish peroxidase (HRP) and alkaline phosphatase (ALP) reactions are limited by substrate turnover rates.
- Surface immobilization inefficiencies: Antibody-antigen interactions are stochastic, leading to suboptimal target capture at ultra-low concentrations.
- Diffusion limitations: At low analyte concentrations, mass transport effects become a critical factor in antigen capture efficiency.
To overcome these barriers, researchers have developed highly sensitive ELISA platforms incorporating molecular amplification, nanotechnology, and computational signal processing.
Enzyme Engineering for Signal Amplification
HRP and ALP Variants with Enhanced Turnover Rates
- Genetic modifications: Engineering hyperactive HRP and ALP variants with increased catalytic efficiencies.
- Polymer-enzyme conjugation: Conjugating multiple enzyme molecules to a single antibody to amplify signal output.
- Metal-enzyme hybrids: Coupling metal ions (e.g., lanthanides) to enzymatic cofactors to improve redox potential and substrate interaction rates.
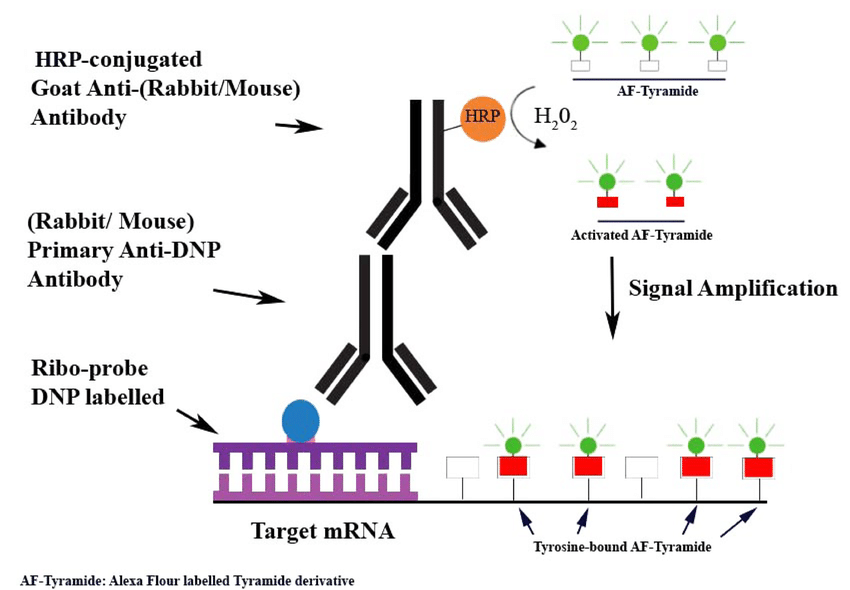
Tyramide Signal Amplification (TSA)
TSA leverages the catalytic activity of HRP to deposit multiple tyramide-conjugated fluorophores or haptens near the detection site, significantly increasing signal intensity.
- Advantages: High localized amplification without excessive background noise.
- Applications: Used in ultra-sensitive immunohistochemistry (IHC) and single-molecule ELISA.
Nanoparticle-Enhanced ELISA
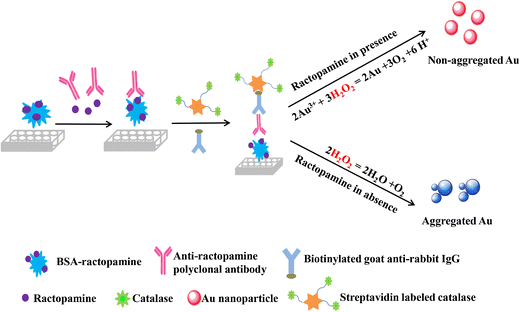
Gold Nanoparticles (AuNPs) for Colorimetric Enhancement
- Plasmonic amplification: AuNPs exhibit surface plasmon resonance (SPR) properties that amplify colorimetric signals in ELISA assays.
- Dual-enzyme conjugation: Coupling AuNPs with HRP/ALP for increased enzymatic turnover.
- Application: Cancer biomarker detection at femtogram sensitivity.
Quantum Dot (QD) Fluorescence-Based ELISA
Quantum dots provide narrow emission spectra, high quantum yields, and photostability, making them superior to traditional organic dyes in fluorescence-based ELISA.
- Multiplexing capability: QDs allow simultaneous detection of multiple analytes via distinct emission wavelengths.
- Single-molecule resolution: QD-based ELISA enables detection at attogram levels.
Magnetic Nanoparticles (MNPs) for Target Enrichment
MNPs functionalized with antibodies enhance target capture by enabling rapid, high-efficiency immunoprecipitation prior to ELISA detection.
- Magnet-assisted washing: Reduces non-specific binding and increases assay specificity.
- Enzyme-loaded MNPs: Enable localized signal amplification at the detection site.
- Application: Ultra-low abundance pathogen detection in virology.
Next-Generation Substrates for ELISA
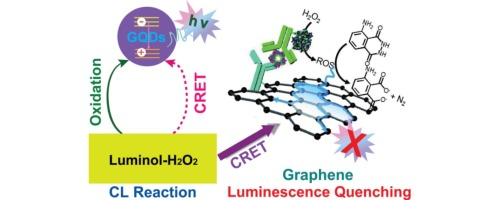
Super-Quenching Chemiluminescent Substrates
Chemiluminescent ELISA (CL-ELISA) benefits from substrate advancements that enhance photon yield and stability.
- Enhanced luminol derivatives: Provide prolonged emission for femtogram sensitivity.
- Peroxide-based cascade reactions: Amplify chemiluminescent signal intensity.
Electrochemical ELISA (EC-ELISA)
Electrochemical detection integrates ELISA with amperometric or voltammetric signal readout, improving sensitivity beyond optical detection methods.
- Redox cycling strategies: Use reversible electron transfer reactions to amplify weak signals.
- Nanostructured electrodes: Improve surface area for enhanced signal generation.
Single-Molecule ELISA (SiM-ELISA)
SiM-ELISA utilizes digital counting of enzyme-labeled antibody-antigen complexes in femtoliter microarrays.
- Digital readout: Single enzyme molecules catalyze reactions in isolated wells, allowing precise quantification at extremely low concentrations.
- Application: Neuroscience research for early-stage detection of Alzheimer’s disease biomarkers.
Computational Signal Processing for Ultra-Low Detection
Advanced machine learning algorithms refine ELISA signal extraction and background correction.
- Deep learning-based deconvolution: Enhances weak signal detection by filtering noise from raw optical and electrochemical data.
- Bayesian inference models: Predict low-abundance analyte concentrations with higher confidence intervals.
- Principal component analysis (PCA) for multiplexed assays: Disentangles overlapping signals in multi-target ELISA.
Future Directions and Implications
- Point-of-care (POC) ultra-sensitive ELISA: Integration of high-sensitivity ELISA with portable microfluidic platforms.
- Single-cell proteomics applications: Enables analysis of low-abundance proteins in individual cells.
- Real-time in vivo monitoring: Developing implantable biosensors with ELISA-like specificity for continuous biomarker tracking.
The evolution of ELISA beyond conventional detection limits has been driven by innovations in enzyme engineering, nanotechnology, chemiluminescent substrates, and digital signal processing. These advances have propelled ELISA sensitivity into the femtogram and attogram range, opening new avenues for early disease detection, precision medicine, and high-resolution immunoassays. As next-generation ELISA technologies continue to develop, their impact will extend into areas such as single-molecule diagnostics, real-time biosensing, and ultra-sensitive biomarker discovery.